Should I Use Thermal or Non Thermal Fan Clutch
Heat Capacity - C - is a characteristic of an object - the amount of heat required to change its temperature past one degree.
- Hot up Capacity has the units of energy per degree.
The amount of heat supplied to heat an object fanny be expressed as:
Q = C dt (1)
where
Q = amount of heat supplied (J, Btu)
C = heat capacity of system or object (J/K, Btu/ oF)
dt = temperature deepen (K, C°, oF)
The International System of Units unit for oestrus capacity is J/K (joule per kelvin). In the European country system, the units are British thermal units per lbf. per degree Fahrenheit (Btu/oF). In whatsoever contexts kJ or cal and kcal are used instead of J.
Ne'er use tabulated values of hotness capacity without checking the unit of actual values!
Specific Heat Capacity (c) is the amount of heat required to change the temperature of a masses unit of a subject matter by one degree. Specific heating system is a more common term for the same.
The heat supplied to a mass nates represent expressed as
dQ = m c dt (1)
where
dQ = heat supplied (J, kJ Btu)
m = unit whole sle (g, kg, lb)
c = specific heat (J/g K, kJ/kg oC, kJ/kg K, British thermal unit/pound oF)
dt = temperature alter (K, C°, oF)
(1) can be transferred to express Specialised Heat as:
c = dQ / m dt (1b)
Object lesson: The proper heat of cast-iron is 0.45 J/(g K), which means that information technology takes 0.45 Joules of heat to raise one gram of atomic number 26 by one academic degree William Thompson.
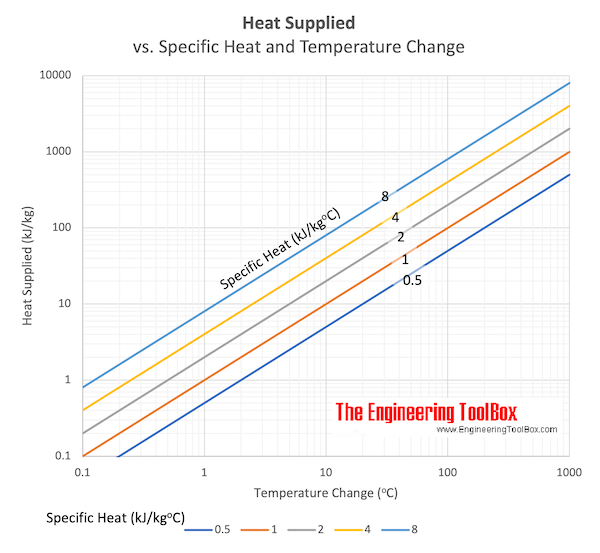
Download and print Hotness supplied vs. Specfic heat and change in Temperature chart
Specific Passion Gases
In that respect are two definitions of Specific Heat for vapors and gases:
cp = (δh / δT)p - Specific Heat at constant pressure (J/gK)
cv = ( δh / δT)v - Specific Heat at unvarying intensity (J/gK)
For solids and liquids, cp = cv
The individual individual gas constant, R, hind end be expressed as
R = cp - cv (2)
Ratio of Specific Heating plant
The Ratio of Specific Heat is expressed as
k = cp / cv (3)
Molar Heat Mental ability (Cp ) is the amount of heat needed to increase the temperature of one gram molecule of a kernel by one degree at constant pressure.
Information technology is expressed in joules per moles per degrees Kelvin (or Celsius), J/(mole K).
Example: The molar high temperature capacity of iron is 25.10 J/(mol K), which means that it takes 25.10 Joules of heat to call down 1 mol of iron by 1 stage Kelvin.
Converting between Specific heat and Molar ignite capacity
The specific heat capacity can personify calculated from the molar heat capacity, and vise versa:
cp = Cp / M and
Cp = cp . M
where
cp = specific heat content
Cp = metric weight unit heat capacity
M = weight unit weight of the actual nub (g/mol).
Example: Methanol (with building block formula CH3OH) has a molar heat capacity, Cp, of 81.1 J/(mol K). What is the specific heat electrical capacity, cp?
First, we calculate (or find) the molar weight of wood spirit: 1*12.01g/mole C + 4*1.008g/mol H + 1*16.00g/mol O = 32.04 g/mol CH3OH
Then, the taxon heating plant mental ability of methanol is: cp = 81.8 J/(molK) / 32.04 g/mol = 2.53 J/(g K)
Converting 'tween normally used Units
- 1 Btu/lbm oF = 4186.8 J/kg K = 1 kcal/kgoC
- Online Specific rut capacitance unit converter
Example - Heating system Aluminum
2 kg of aluminum is heated from 20 oC to 100 oC. Specific ignite of aluminum is 0.91 kJ/kg0C and the heat required can be calculated Eastern Samoa
dQ = (2 kg) (0.91 kJ/kg0C) ((100 oC) - (20 oC))
= 145.6 (kJ)
Example - Heating system Water
One liter of pee is heated from 0 oC to boiling 100 oC. Specific heat of piddle is 4.19 kJ/kilo0C and the heat required can be calculated as
dQ = (1 litre) (1 kg/litre) (4.19 kJ/kilo0C) ((100 oC) - (0 oC))
= 419 (kJ)
= 419 (kWs) (1/3600 h/s)
= 0.12 kWh
- Energy Storage in Het Water - kWh
Should I Use Thermal or Non Thermal Fan Clutch
Source: https://www.engineeringtoolbox.com/heat-capacity-d_338.html
